Overview of Grey, Blue, and Green Hydrogen
- Theo Pascal
- May 16, 2022
- 10 min read
Updated: May 31, 2022

Introduction
Hydrogen is the most abundant element on Earth and is mainly found in water and organic compounds (Dawood, et al., 2020). With a high energy density of 33.3 kWh/kg and a low volumetric density of 0.09 kg/m3 at normal conditions (Ludwig Bölkow Systemtechnik, n.d.), hydrogen is considered to have a very high potential as an energy carrier due to its potential to be compressed into smaller, and more transportable, volumes.
On top of this, hydrogen can be converted into electricity while only emitting heat and water vapor, thus no harmful by-products are produced (ADFC, n.d.). As such, hydrogen has the potential to play a major role in the movement towards a sustainable, net-zero future.
The main concern and uncertainty with the mass adoption of hydrogen is its method of production. Currently, almost all hydrogen is produced from fossil fuels – 76% of which come from the steam reforming of natural gases and the remainder from the gasification of coal. In total, this produces around 830 MtCO2/annum (Cavana & Leone, 2021).
Fortunately, technologies such as carbon capture and storage (CSS) and electrolyzers are expected to increase in usage due to their ability to lower the environmental impact of hydrogen production. However, as these technologies are still in their infant stage, the production costs are much higher than that of fossil fuel-based hydrogen therefore, the adoption of these technologies is not considered feasible until around 2030.
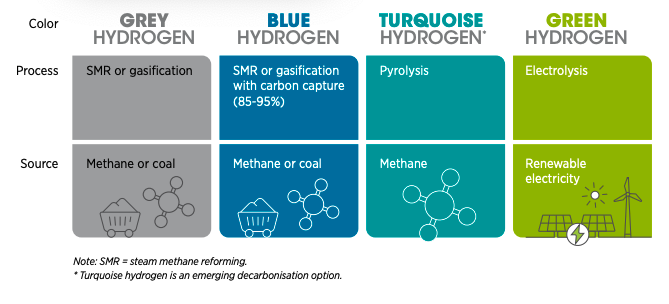
There are various types of hydrogen classified by their method of production and distinguished by ‘color’. The main types of hydrogen under consideration are grey hydrogen, blue hydrogen, and green hydrogen. Each of which is discussed further below, along with an overview of hydrogen storage and transportation methods.
Grey Hydrogen
Generally, the term “grey hydrogen” is used to describe hydrogen produced from fossil fuels. The most common form of grey hydrogen is that which originates from natural gas and is extracted using processes such as ‘steam methane reforming (SMR) and ‘autothermal reforming’ (ATR). These processes extract hydrogen from hydrocarbons by splitting natural gases into hydrogen and CO2 (Petrofac, 2021).
Steam Methane Reforming
Focusing on SMR, hydrogen is extracted from methane as it reacts with high-temperature steam, at around 700-930 ℃ and pressures of around 3-25 bar, along with a catalyst (EIA, n.d.). The efficiency of SMR generally ranges between 65% and 85% and is thus in the higher range of most commercial hydrogen production technologies (Kayfeci, 2019).
SMR is also the most widespread production process of grey hydrogen while also being the least expensive with cost estimates of around EUR 1.5/kg (Kalamara & Efstathiou, 2013; European Commission, 2020).
However, this cost is expected to increase with the cost of natural gas and carbon tax. Typically, around 4.5 m3 of natural gas is required per kg of hydrogen produced (Milbrant & Mann, 2009). Grey hydrogen also comes with a major disadvantage of high carbon emissions; typically, around 9.3 kg of CO2 per kg of hydrogen is produced (Giovannini, 2020).
It is thus an interest to transition the production of grey hydrogen to more environmentally friendly solutions such as green and blue hydrogen.
Blue Hydrogen
Blue hydrogen refers to hydrogen produced from fossil fuels, with the same production processes as grey hydrogen, but with the addition of carbon capture and storage (CCS) to offset the levels of carbon dioxide released into the atmosphere. It is not possible to capture all the carbon dioxide through CCS, therefore, blue hydrogen is referred to as a ‘low-carbon hydrogen’ alternative (National Grid, 2021a).
Steam Methane Reforming and Carbon Capture and Storage
Through CCS, CO2 emissions are captured and stored underground, often in salt caverns or depleted oil and gas reserves, rather than dispersed into the atmosphere (Hague, 2021). Typically, around 80-90% of the CO2 emissions can be captured, thus still emitting around 10-20%. Therefore, we can expect CO2 emissions of around 1.4 kg of CO2 per kg of blue hydrogen produced (Giovannini, 2020).
This added CCS technology also introduces additional technical challenges as well as an increase in price with blue hydrogen cost estimates of around EUR 2/kg (Hague, 2021; European Commission, 2020).
This cost includes the CAPEX, OPEX, carbon tax, and natural gas prices (Global CCS Institute, 2021) in which fuel is the largest cost component, accounting for 45-75% of production costs (KPMG, 2021). Similar cost estimates assume a natural gas price of around EUR 7/GJ (Glabal CSS Institute, 2021).
As a result of increased natural gas prices and carbon taxes, the cost of blue hydrogen is expected to increase to EUR 2.35/kg in 2025 and to EUR 2.70/kg in 2035 (Deloitte, 2020). See Table 1 for a cost summary with the 2025 cost determined through extrapolation.

Green Hydrogen
Green hydrogen also referred to as renewable hydrogen (European Commission, 2020), is the cleanest form of hydrogen in which no greenhouse gases are emitted throughout the production process of electrolysis. This involves the splitting of water molecules into their constituent atoms of oxygen and hydrogen.
For the process to be fully ‘green’, the electrolysis is powered by clean energy from renewable energy sources such as wind and solar power (National Grid, 2021b). At present, only around 1% of all hydrogen production is in the form of green hydrogen (Giovannini, 2020).
Electrolysis
Electrolysis occurs inside an electrolyzer. This consists of an anode and cathode, separated by an electrolyte. These collect the individual elements through attraction whereby the positively charged hydrogen ions are attracted to the negatively charged cathode while the negatively charged oxygen ions are attracted to the positively charged anode (Bruce, et al., 2018), see Figure 1.

Typically, around 9 liters of water and 48 kWh are required for every kg of green hydrogen produced. Therefore, it is important to have an appropriate water supply when deciding on the location of an electrolyzer as well as a supply of renewable energy.
Furthermore, the purity of water is also important to minimize side reactions caused by ions found in naturally occurring water, such as salt (Bruce, et al., 2018; Antweiler, 2020). As such, the use of fresh water and seawater should be considered.
There are various types of electrolyzers; examples of such technologies are polymer electrolyte membrane (PEM) electrolyzers, alkaline electrolyzers, and solid oxide electrolyzers. Each differs based on the electrolyte material and the type of reactions that occur (U.S. Department of Energy, 2021) while also providing different benefits and challenges.
While both are mature technologies, studies indicate that the hydrogen industry will mostly depend on PEM electrolyzers over alkaline electrolyzers due to their compact design, high system efficiency, fast response times, dynamic operations, low temperatures, and their ability to produce ultrapure hydrogen at raised pressures of around 30-80 bar (Khan, et al., 2021).
However, it should be noted that there are also new emerging electrolysis technologies such as solid oxide electrolyzers that could overtake the more established technologies with further development (Mathiesen, et al., 2013).
Polymer Electrolyte Membrane Electrolysers
PEM electrolyzers have a solid specialty plastic material for the electrolyte. Currently, the most advanced PEM electrolyzers can produce hydrogen at 400m3/h, however, further development of such technology is restricted by high manufacturing costs (Guo, et al., 2019).
PEM electrolyzers do however have fast start-up times, minimal corrosion, simple maintenance, and few components (U.S. Department of Energy, 2021).
Alkaline Electrolysers
Alkaline electrolyzers use a liquid alkaline solution, such as sodium or potassium hydroxide, as the electrolyte (Cummins, 2020). The technology behind alkaline electrolyzers is well established with low manufacturing costs.
With a potential hydrogen production of 1000m3/h, these electrolyzers are suitable for large-scale hydrogen production. In contrast to the PEM electrolyzers, however, alkaline electrolyzers have a slow start-up, vulnerable to corrosion, complicated maintenance, and consist of many components (Guo, et al., 2019).
Solid Oxide Electrolysers
Solid oxide electrolyzers use a solid ceramic material as the electrolyte. Solid oxide electrolyzers operate at much higher temperatures, at around 700-800℃, while PEM electrolyzers operate at 80-90℃ and alkaline electrolyzers at up to 100℃ (Cummins., 2020; U.S. Department of Energy, 2021).
Seawater Desalination
Freshwater can be bought from suppliers however, the usage of seawater fed electrolysis a growing as it can be more efficient and appropriate depending on the potable water scarcity within the location of the plant. Representing around 96.5% of the water on Earth, seawater can be considered in abundance and particularly suitable for coastal regions.
The purification and desalination of water can be achieved through various methods such as reverse osmosis, multi-stage flash distillation, electrodialysis, and multiple effect distillation (Ibrahim & Moussab, 2020; Khan, et al., 2021).
Seawater reverse osmosis (SWRO) has undergone great technological advancements in the form of improved membrane technology, more efficient energy recovery devices, and process optimization that have resulted in lower energy, CAPEX, and OPEX requirements.
Desalination plants can expect power consumption of about 3 kWh per m3 of desalinated water. The CAPEX of an SWRO plant varies depending on the technology, location, and plant size (Khan, et al., 2021). The CAPEX of various plant sizes is summarised in Table 2.
The OPEX of an SWRO plant accounts for plant maintenance, labor, chemicals, and membrane exchange and equates to around EUR 0.23/(m3/annum) (Azinheira, et al., 2019). Based on the typical area requirement of 25 acres for a seawater desalination plant with a capacity of 100 million m3/annum (Einav, et al., 2002), the required land area can be considered at 0.01 km2/(10 million m3/annum).

REFERENCES:
ADFC (no date). Hydrogen Basics. [online] Available at: https://afdc.energy.gov/fuels/hydrogen_basics.html. [Accessed 20 October 2021].
Antweiler, W. (2020). What role does hydrogen have in the future of electric mobility? [online] Available at: https://wernerantweiler.ca/blog.php?item=2020-09-28. [Accessed 12 November 2021].
Azinheira, G., Segurado, R. & Costa, M. (2019). ‘Is Renewable Energy-Powered Desalination a Viable Solution for Water Stressed Regions? A Case Study in Algarve, Portugal’, Energies, Vol. 12, doi: 10.3390/en12244651.
Bezdek, R. H. (2019). ‘The hydrogen economy and jobs of the future’, Renew. Energy Environ. Sustain., Volume 4, January 2019, DOI: https://doi.org/10.1051/rees/2018005.
BNEF (2020). ‘Hydrogen Economy’ Offers Promising Path to Decarbonization. [online] Available at: https://about.bnef.com/blog/hydrogen-economy-offers-promising-path-to-decarbonization/. [Accessed 22 December 2021].
Bruce, S., Temminghodd, M, Hayward, J., Schmidt, E., Munnings, C., Palfreyman, D. & Hartley, P. (2018). ‘Australia’s National Hydrogen Roadmap’, CSIRO, Energy and Futures, Australia.
Cavana, M. & Leone, P. (2021). ‘Solar Hydrogen from North Africa to Europe through Greenstream: A simulation-based analysis of blending scenarios and production plant sizing’, International Journal of Hydrogen Energy, Vol. 46, pp. 22618-22637.
Cummins Inc. (2020). Electrolyzers 101: What they are, how they work and where they fit in a green economy. [online] Available at: https://www.cummins.com/news/2020/11/16/electrolyzers-101-what-they-are-how-they-work-and-where-they-fit-green-economy. [Accessed 20 October 2021].
Dawood, F., Anda, M. & Shafiullah, G. M. (2020). ‘Hydrogen production for energy: An overview’, International Journal of Hydrogen Energy, Vol. 45, Issue. 7, February 2020, pp. 3847-3869.
Deloitte (2020). ‘Investing in hydrogen – Ready, set, net zero’, November 2020.
Duren, M. (2017). ‘Energy in Times After the Energy Transition’, Understanding the Bigger Energy Picture, DOI 10.1007/978-3-319-57966-5_3, pp.45-87.
European Commission (2020). ‘A hydrogen strategy for a climate-neutral Europe’, Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, Brussels, July 2020.
EIA (no date). Hydrogen explained- Production of hydrogen. [online] Available at: https://www.eia.gov/energyexplained/hydrogen/production-of-hydrogen.php. [Accessed 16 December 2021].
Einav, R., Harussi, K. & Perry, D. (2002). ‘The footprint of the desalination processes on the environment’, Desalination, Vol. 152, pp.141-154.
EERE (2021). Liquid Hydrogen Delivery. [online] Available at: https://www.energy.gov/eere/fuelcells/liquid-hydrogen-delivery. [Accessed 20 October 2021].
Giovannini, S. (2020). 50 shades of (grey and blue and green) hydrogen. [online] Available at: https://energy-cities.eu/50-shades-of-grey-and-blue-and-green-hydrogen/. [Accessed 03 December 2021].
Guo, Y., Li, G., Zhou, J. & Liu, Y. (2019). ‘Comparison between hydrogen production by alkaline water electrolysis and hydrogen production by PEM electrolysis’, Earth and Environmental Science, Vol. 371, 2019, doi: 10.1088/1755-1315/371/4/042022.
Hague, O. (2021). What are the 3 Main Types of Hydrogen? [online] Available at: https://www.brunel.net/en/blog/renewable-energy/3-main-types-of-hydrogen. [Accessed 03 December 2021].
Ibeh, B., Gardner, C. & Ternan, M. (2007). ‘Separation of hydrogen from a hydrogejn/methane mixture using a PEM fuel cell’, International Journal of Hydrogen Energy, Vol. 32, Issue 7, May 2007, pp. 908-914.
Ibrahim, J. M. & Moussab, H. (2020). ‘Recent advances on hydrogen production through seawater electrolysis’, Materials Science for Energy Technologies, Vol. 3, 2020, pp. 780-807.
IEA (2021a). Global Hydrogen Review 2021. [online] Available at: https://www.iea.org/reports/global-hydrogen-review-2021/executive-summary. [Accessed 22 December 2021].
IEA (2021b). Net Zero by 2050 – A Roadmap for the Global Energy Sector. [online] Available at: https://www.iea.org/reports/net-zero-by-2050. [Accessed 14 December 2021].
Jeffers, B., Gutcher, S., Hassan, N., Pace, S. & Hoogendoorn, R. (2021). Hydrogen: Ready for Take Off?, University of Surrey, Multi-Disciplinary Design Project, 2020-21.
Kalamara, C. M. & Efstathiou, A. M. (2013). ‘Hydrogen Production Technologies: Current State and Future Developments’, Power Options for the Eastern Mediterranean Region, Conference Papers in Energy, November 2012, Limassol, Cyprus.
Khan, M. A., Al-Attas, T., Roy, S., Rahman, M. M., Ghaffour, N., Thangadurai, V., Larter, S., Hu, J., Ajayan, P. M. & Kibria, M. G. (2021). ‘Seawater electrolysis for hydrogen production: a solution looking for a problem?’, Energy & Environmental Science, Vol. 14, Issue 9, pp. 4831-4839.
KPMG (2021). The Hydrogen Trajectory. [online] Available at: https://home.kpmg/xx/en/home/insights/2020/11/the-hydrogen-trajectory.html. [Accessed 22 December 2021].
Ludwig Bölkow Systemtechnik (no date). Hydrogen Data. [online] Available at: http://www.h2data.de/. [Accessed 20 October 2021].
Mathiesen, B. V., Ridjan, I., Connolly, D., Nielsen, M. P., Vang Hendriksen, P., Bjerg Mogensen, M., Hojgaard Jensen, S. & Dalgaard Ebbesen, S. (2013).
Technology data for high temperature solid oxide electrolyser cells, alkali and PEM electrolysers, Department of Development and Planning, Aalborg University.
McMahon, M. (2020). New Technology Seamlessly Converts Ammonia to Green Hydrogen. [online] Available at: https://www.sciencedaily.com/releases/2020/11/201118141718.htm. [Accessed 20 October 2021].
Melaina, M. W., Antonia, O. & Penev, M. (2013). ‘Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues’, NREL, technical report, March 2013.
Milbrandt, A. & Mann, M. (2009). ‘Hydrogen Resource Assessment – Hydrogen Potential from Coal, Natural Gas, Nuclear, and Hydro Power’, NREL, Technical Report, February 2009.
National Grid (2021a). The hydrogen colour spectrum. [online] Available at: https://www.nationalgrid.com/stories/energy-explained/hydrogen-colour-spectrum. [Accessed 09 October 2021].
National Grid (2021b). What is hydrogen? [online] Available at: https://www.nationalgrid.com/stories/energy-explained/what-is-hydrogen. [Accessed 09 October 2021].
Petrofac (2021). The difference between green hydrogen and blue hydrogen. [online] Available at: https://www.petrofac.com/media/stories-and-opinion/the-difference-between-green-hydrogen-and-blue-hydrogen/. [Accessed 03 December 2021].
PwC (2021). The green hydrogen economy – Predicting the decarbonisation agenda of tomorrow. [online] Available at: https://www.pwc.com/gx/en/industries/energy-utilities-resources/future-energy/green-hydrogen-cost.html. [Accessed 22 December 2021].
Statkraft (2021). Green Ammonia: Clime Friendly Fuel for Long Distances and Heavy Tasks. [online] Available at: Green ammonia: Climate-friendly fuel for long distances and heavy tasks (statkraft.com). [Accessed 20 October 2021].
U.S. Department of Energy (2021). Hydrogen Production: Electrolysis. [online] Available at: https://www.energy.gov/eere/fuelcells/hydrogen-production-electrolysis. [Accessed 18 October 2021].
Vickers, J., Peterson, D. & Randolph, K. (2020). ‘Cost of Electrolytic Hydrogen Production with Existing Technology’, DOE Hydrogen and Fuel Cells Program Record, Department of Energy United States of America, September 2020.
Wang, A., Jens, J., Mavins, D., Moultak, M., Schimmel, M., Leun, K., Peters, D. & Buseman, M. (2021). ‘Analysing future demand, supply, and transport of hydrogen’, European Hydrogen Backbone, Guidehouse, June 2021.
Wijk, A. V. & Chatzimarkakis, J. (2020). ‘Green Hydrogen for a European Green Deal – A 2x40 GW Initiative’, Hydrogen Europe, March 2020.
Wood Mackenzie (2020). Hydrogen production costs to 2040: Is a tipping point on the horizon? [online] Available at: https://www.woodmac.com/our-expertise/focus/transition/hydrogen-production-costs-to-2040-is-a-tipping-point-on-the-horizon/?utm_campaign=energy-transition&utm_medium=article&utm_source=gtm&utm_content=hydrogen-costs. [Accessed 22 December 2021].
Zumdahl, S. S. (2020). Ammonia – Chemical Compound. [online] Available at: ammonia | Definition & Uses | Britannica. [Accessed 20 October 2021].